Saffron
A Review by John C. Leffingwell, Ph.D.
Saffron is considered to be the worlds most expensive spice. The flower of Crocus sativa is a light purple, but it is the thread-like reddish colored stigma of the flower that is valued both as a spice and as a natural colorant. Saffron is hand harvested in the autumn, and the stigma is laboriously separated to yield the reddish colored spice. It takes in excess of 70,000 flowers to yield just one pound (0.45 kilo) of saffron spice. The odor of saffron is sometimes described as like the “sea” air. The natural color is a powerful yellow in applications such as for saffron rice.
Saffron Flower
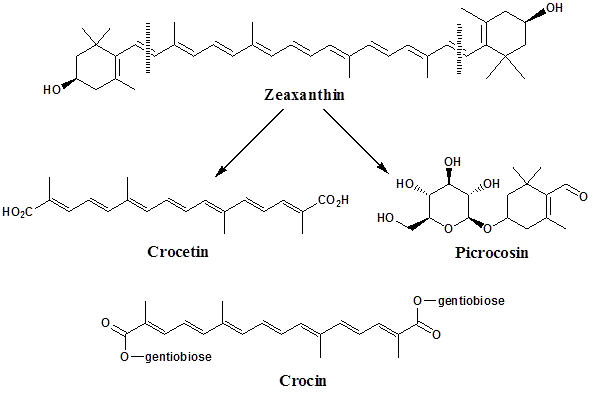
While the color is mainly due to the degraded carotenoids (crocin and crocetin), the flavor comes from the carotenoid oxidation products (mainly safranal and the bitter glucoside picrocrocin). It was proposed by Pfander & Schurtenberger (1982) that the biogenesis of the color principles and odor active compounds is derived by bio-oxidative cleavage of Zeaxanthin at the points indicated.
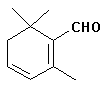
As mentioned, the major organoleptic principle present is “Safranal” [2,6,6-Trimethylcyclohexa-1,3-dien-1-carboxaldehyde], which is easily formed by de-glucosylation of picrocrocin. Safranal composes as much 70% of total volatiles.
Safranal…..
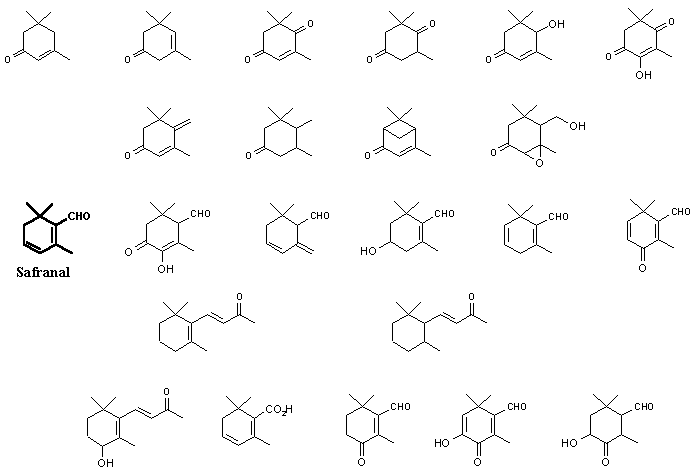
Among the other carotenoid derived volatiles found by various workers (see references below) are the following materials:
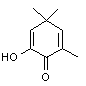
In a recent series of analyses carried out in 1997 (comparing various methods of volatiles isolation), Tarantilis and Polissiou implied that some isolates (such as those on the bottom row, above) may be artifacts formed during a steam distillation isolation procedure. In their studies they confirmed that Safranal was the major component, comprising about 70% of total volatiles. Cadwallader, et.al., in 1997 reported on the aroma dilution analysis of saffron volatiles and found that a minor component, 2-Hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one (also isolated by Tarantilis and Polissiou from steam distillation of Greek red saffron), was the most powerful aroma constituent of saffron, followed by safranal. Its odor was descibed as “saffron, dried hay like”. Interestingly, 2-Hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one is also the unique pheromone from the bark beetle, Ips pini known as Lanierone (Teale, et. al.).
۲-Hydroxy-4,4,6-trimethyl-2,5-cyclohexadien-1-one
Aroma: saffron, dried hay like
Saffron References:
Alonso, G. L.; Salinas, M. R.; Esteban-Infantes, F. J.; Sanchez Fernandes, M. A. Determination of safranal from saffron (Crocus sativus; L.) by thermal desorption-gas chromatography. J. Agric. Food Chem. 1996, 44, 185
Basker, D.; Negbi, M. Uses of Saffron. Econ. Bot. 1983, 37, 228-236.
Bucheker, R.; Eugster, C. H. Absolute configuration of picrocrocin. Helv. Chim. Acta 1973, 56, (3), 1121-1125.
Cadwallader, K.R.; Back, H.H.; Cai, M.; ACS Symposium Series, No. 166 (Spices), American Chemical Society, Pub., Washington DC, 1997, pp.66-79.
Castellar, M. R.; Montijano, H.; Manjon, A.; lborra, J. L. Preparative high-performance liquid chromatographic purification of saffron secondary metabolites. J. Chromatogr. 1993, 648, 187-190.
Corradi, C.; Michelli, G. Determinazione spectrofotometrica del potere colorante, americane ed odoroso dello zafferano. Boll. Chim. Farm. 1979a, 118, 553-562.
Crouzet, J.; Kanasawud, P. Formation of Volatile Compounds by Thermal Degradation of Carotenoids. In Methods in Enzymology; Packer, L., Ed.; Academic Press: London, San Diego, New York, Boston, Sydney, Tokyo, and Toronto, 1992; Vol. 213, Chapter 7.
Curro, P.; Lanuzza, F.; Micali, G. Volutazione della frazione volatile dello zafferano mediante gascromatografia dello spazio di testa. Rassegna Chim. 1986, 6, 331-334.
Curro, P.; Micali, G.; Lanuzza, F. Lo zafferano: un colorante ed aromatizzante naturale; XI Congresso Nazionale di Merceologia, Napoli, Italy, 1984, pp 490-501.
Himeno, H.; Sano, K. Synthesis of crocin, picrocrocin and safranal by saffron stigma-like structures proliferated in vitro. Agric. Biol. Chem. 1987, 51 (9), 2395-2400.
Kanasawud, P.; Crouzet, J. C.; Mechanism of Formation of Volatile Compounds by Thermal Degradation of Carotenoids in Aqueous Medium. 1. beta-Carotene Degradation. J Agric. Food Chem. 1990, 38, 237-243.
Kanasawud, P.; Crouzet, J. C.; Mechanism of Formation of Volatile Compounds by Thermal Degradation of Carotenoids in Aqueous Medium. 2. Lycopene Degradation. J. Agric. Food Chem. 1990, 38, 1238-1242,
lborra, J. L.; Castellar, M. R.; CAnovas, M.; Manj6n, A. TLC preparative purification of picrocrocin, HTCC and crocin from saffron, J. Food Sci. 1992, 57 (3), 714-716, 731.
Mikami, Y; Fukunaga, Y.-, Arita, M; Obi, Y.; Kisaki, T. Preparation of aroma compounds by microbial transformation of isophorone with Aspergillus niger. Agric. Biol, Chem. 1981, 45, 791-793.
Mookherjee, B. D.; Trenkle, R. W.; Wilson, R. A. Live vs Dead. Part II. A Comparative Analysis of the Headspace Volatiles of Some Important Fragrance and Flavor Raw Materials. J. Essent. Oil Res. 1987, 2, 85-90.
Morimoto, S.; Umezaki, Y.; Shoyama, Y.; Saito, H.; Nishi, K; Irino, N. Post-harvest degradation of carotenoid glucose ester in saffron. Planta Med., 1994, 60, 438-440
Morjam, H.; Tarantilis, P.; Polissiou, M.; Manfait, M. Growth inhibition and induction of erythroid differentiation activity by crocin, dimethylerocetin and beta-carotene on K562 tumor cells. Anticancer Res. 1990, 10, 1398-1399.
Nair, S. C.; Salomi, M. J.; Varghese, C. D.; Panikkar, B.; Panikkar, K. R. Effect of saffron on thymocyte proliferation, intracellular glutathione levels and its antitumor activity. BioFactors 1992, 4, 51-54.
Narasimhan, H.; Chand, H.; Rajalakshmi, D. Saffron: quality evaluation by sensory profile and gas chromatography. J. Food Qual. 1992, 15, 303-314.
Oberdieck, R. Current knowledge and analysis of saffron (Crocus sativus L.). Dtsch. Lebensm. Rundsch. 1991, 87, 246-252,
Pfander, H.; Schurtenberger, H.; Biosynthesis of C20-carotenoids in Crocus sativus. Phytochemistry 1982, 21, 1039-1042.
Pfander, H.; Rychener, M. Separation of crocetin glycosyl esters by high-perfbrmance liquid chromatography. J. Chromatogr. 1982, 234, 443-447.
Pfander, H.; Wittwer, F. Carotenoid glycosides. Part 2. Studies on the carotenoid composition of saffron. Helu. Chim. Acta 1975a, 58, 1608-1620.
Pfander, H.; Wittwer, F. Carotenoid glycosides. Part 3. Studies on the carotenoid composition of saffron. Helv. Chim. Acta 1975b, 58, 2233-2236.
Pfister, S.; Meyer, P.; Steck, A.; Pfander, H. Isolation and structure elucidation of carotenoid-glycosyl esters in Gardenia fruits (Gardenia jasminoides Ellis) and Saffron (Crocus sativus Linne). J. Agric. Food Chem. 1996, 44, 2612-2615.
Rodel, W.; Petrzika, M. Analysis of the Volatile Components of Saffron, J. High-Resolution Chromatogr. 1991, 14, 771-774.
Straubinger, M.; Jezussek, M.; Waibel, R. and Winterhalter, P.; Novel glycosidic constituents of saffron. J. Agric. Food Chem., 1997, 45, 1678-1681.
Sujata, V.; Ravishankar, G. A.; Venkataraman, L. V. Methods for the analysis of the saffron metabolites crocin, crocetins, picrocrocin and safranal for the determination of the quality of the spice using thin-layer chromatography, high-performance liquid chromatography and gas chromatography., J. Chromatogr. 1992, 624, 497-502.
Tarantilis, P. A.; Polissiou, M.; Manfait, M.; Separation of picrocrocin, cis-trans crocins and safranal of saffron using high-performance liquid chromatography with photodiode-array detection. J. Chromatogr. 1994, 664, 55-61.
Tarantilis, P. A.; Polissiou, M.; Morjam, H.; Avot, A.; Bel Jebbar, A.; Manfait, M. Anticancer activity and structure of retinoic acid and carotenoids of Crocus sativus L. on HL60 cells. Anticancer Res. 1992, 12, 1889.
Tarantilis, P. A.; Tsoupras G.; Polissiou. M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV- visible photodiode-array detection-mass spectrometry. J. Chromatogr. 1995, 699, 107-118.
Tarantilis, P. A.; Polissiou, M.; Isolation and identification of the aroma constituents of saffron (Crocus sativa). J. Agric. Food Chem. 1997, 45, 459-462.
Teale; Stephen A.; Webster; Francis X.; Zhang; Aijun, New aggregation pheromone for the bark beetle Ips pini and uses thereof , United States Patent 5,167,955, December 1, 1992
Tsimidou, M.; Tsatsaroni, E. Stability of saffron pigments in aqueous extracts. J. Food Sci. 1993, 58, 1073-1075
Visvanath, S.; Ravishankar, G. A.; Venkataraman, L. V. Induction of crocin, crocetin, picrocrocin and safranal synthesis in callus cultures of saffron Crocus sativus L. Biotechnol. Appl. Biochem. 1990, 12, 336-340.
Zarghami, N. S.; Heinz, D. E. The volatile constituents of saffron. Lebensm. Wiss. Technol. 1971, 4 (2), 43-45.
Zarghami, N. S.; Heinz, D. E.; Monoterpene aldehydes and isophorone-related compounds of saffron. Phytochemistry 1971, 10, 2755-2761.
https://medplant.ir/?p=8869